Research progress on surface modification of high nickel ternary cathode materials for lithium ion batteries
Release time:
2024-06-07
With the gradual scarcity of fossil energy and the increasingly severe environmental pollution, the research and development of high-performance electrochemical energy storage devices is urgent. Among the many energy storage devices, lithium-ion battery (LIB) has become the most important energy source for electric vehicles and portable electronic devices because of its high energy density, long cycle life and high energy conversion efficiency. Among the lithium-ion battery cathode materials, the high-nickel ternary cathode materials NCM / NCA ( LiNi xCoyMnzO2 and LiNi xCoyAl zO2 , x y z = 1) have great advantages in energy density and are the dominant materials in the power battery market.
With the gradual scarcity of fossil energy and the increasingly serious environmental pollution, the research and development of high-performance electrochemical energy storage devices has become a matter of urgency. Among many energy storage devices, lithium-ion battery (LIB) has become the main energy source for electric vehicles and portable electronic devices due to its high energy density, long cycle life and high energy conversion efficiency. Among the cathode materials for LIBs, high-nickel ternary cathode materials NCM / NCA ( LiNi xCoyMnzO2 and LiNi xCoyAl zO2 , x+y+z = 1) have a huge advantage in energy density, and are the dominant materials in the power battery market.。
Taking NCM as an example, it has α-NaFeO2-type layered structure, hexagonal crystal system, and R-3m space group. Ni 2+, Co 3+ and Mn 4+ occupy the centre of the octahedron together, and form the layered arrangement by cubic dense stacking. The development of high-nickel ternary cathode materials can meet the increasing demand for energy density. However, there are some deficiencies in high nickel ternary materials, including unstable surface structure, lithium-nickel mixed arrangement, and intergranular cracks.
In order to solve the above problems, researchers have proposed various modification strategies, mainly including surface capping, intracrystalline doping and crystal morphology control, which show good results in improving the electrochemical properties of ternary materials. Among them, surface modification is one of the most commonly used and effective methods. At present, the selected cladding materials for surface modification of high-nickel ternary materials mainly include electrochemically inert materials, ionic conductive materials, and electronic conductive materials, and on the basis of which they are developed to composite cladding.
This paper reviews the research progress of surface modification of high-nickel ternary materials, introduces the protection mechanism of different types of cladding materials and the influence on the electrochemical properties of materials, then analyses the advantages and problems of various cladding materials, and looks forward to the future development trend of cladding modification of high-nickel ternary cathode materials.
The electrochemically inert materials mainly include metal oxides, metal fluorides and metal phosphates, which can effectively block the direct contact between the cathode material and electrolyte, and help to prevent the erosion of HF and the occurrence of interfacial side reactions.
1.1 Metal Oxide Coating
Metal oxide cladding materials mainly include Al 2O3, ZrO2, TiO2, WO3 and so on. The metal oxide capping layer can be converted into metal fluoride by reacting with HF to eliminate HF, thus reducing the acidity of the electrolyte and improving the structural stability of the electrode. However, the relatively low Li+ transport rate and electronic conductivity of these oxides may increase the resistance to electron and ion transport at the capping interface.
Al 2O3 is the most commonly used metal oxide cladding material, and Wu et al. used a polymer-assisted sol-gel method to construct a microporous polymer/γ-Al 2O3 protective layer on the surface of NCM622. This coating layer can effectively reduce the occurrence of side reactions at the electrode-electrolyte interface of NCM622, and significantly improve the electrochemical performance of the material under high-voltage cycling, with its cycling stability and multiplicity performance improved by 22.8% and 26%, respectively, compared with the original material. Ma et al. used hydrothermal synthesis to prepare NCM622 single-crystal particles, and then formed Al 2O3 cladding material by dry-mixing and sintering with triisopropoxyaluminium as the aluminium source. The 13 nm thick Al 2O3 coating layer greatly improves the discharge specific capacity, multiplicity and cycling performance of NCM622, but the lithium storage performance of NCM622 will be reduced by the over-thick Al 2O3 coating layer.
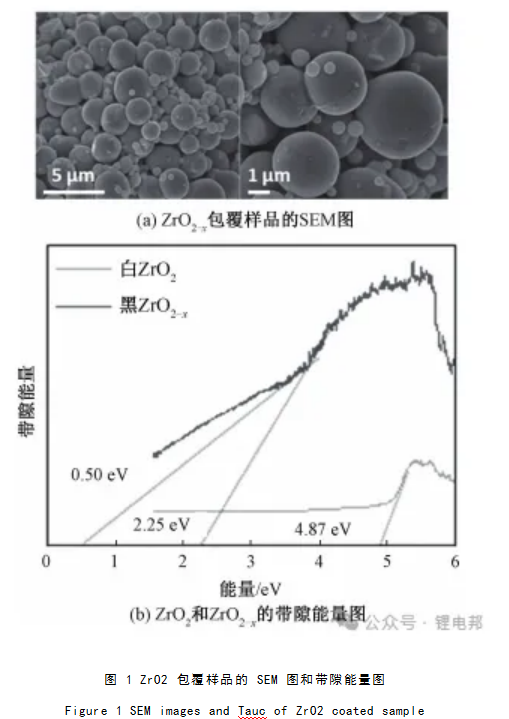
ZrO2 has high chemical stability, and ZrO2 coating can effectively alleviate the decomposition of electrolyte, Kim et al. gave the SEM and band gap energy diagrams of ZrO2-coated samples, as shown in Fig. 1. It can be seen that the white monoclinic ZrO2 is converted to black oxygen-deficient tetragonal ZrO2-x through a simple reduction reaction, which reduces the band energy of the material (Fig. 1(b)) and successfully modifies it on the surface of high-nickel cathode NCM811 (Fig. 1(a)). The black ZrO2- x effectively suppressed the gas precipitation during high-voltage 4.5 V charging by inductively maintaining the high oxidation of Ni 2+ .
TiO2 has been used as a capping material due to its electrochemical inactivity and charge compensation.Mo et al. introduced TiO2 onto the surface of secondary particles of NCM622 samples by wet capping.TiO2 reacted with residual lithium compounds to form Li 2TiO3 and acted as a spacer layer, which reduced the occurrence of side reactions. In addition, diffusion layers with different Ti 4+ concentrations from the outside to the inside were obtained, which not only strengthens the primary particles and reduces the gaps between randomly oriented grains, but also provides a coating layer that helps to diffuse Ti 4+ into the lattice of the NCM622, thereby increasing the lattice spacing and allowing for easier subsequent Li+ migration, an increase in the rate of migration, and a higher level of mechanical strength and interfacial stability. stability will also be higher. As a result, the cycling stability of the NCM622 samples is enhanced.
WO3 has a high electronic conductivity (1.76 S-cm - 1 ), and as an acidic oxide, it has better resistance to HF etching. In addition, WO3 can react with lithium compounds, which can help to eliminate some of the alkaline compounds left on the surface of NCM materials.Gan et al. dissolved a certain amount of WO3 in H2O2 and dispersed it in anhydrous ethanol, and then mixed it with NCM811 to evaporate and high-temperature sintering to form the WO3 cladding. The results showed that the WO3 coating modification reduced the polarisation of NCM811 to a certain extent and improved the multiplicity and cycling performance of NCM811.
In addition, SiO2 has attracted much attention due to its low electrochemical activity, abundant reserves, environmental friendliness, and low price, etc. It can also react with HF to protect the cathode particles from the erosion of electrolyte and mitigate the surface structure degradation during cycling. It can also react with HF to protect the cathode particles from electrolyte erosion and alleviate the surface structure degradation during the cycling process.Li et al. used the electrostatic gravitational method to uniformly adsorb the SiO2 sol on the surface of NCM715 by adjusting the electrodynamic potential between the SiO2 sol suspension and NCM715, and then formed the SiO2 overlayer after heat treatment.SiO2 overlayer on the surface of NCM715 reduces the reaction between the electrolyte and the anode, and protects the surface structure of NCM715 by reducing the reaction between the electrolyte and the anode. The SiO2 capping layer on the surface of NCM715 reduces the reaction between the electrolyte and the anode, protects the laminar structure of the electrode, reduces the interfacial impedance, and exhibits good electrochemical stability even at a high cut-off voltage of 4.5 V.
1.2 Metal Fluoride Capping Materials
The most important metal fluoride capping material is AlF3, which can inhibit lithium-nickel mixing and lithium loss during the cycling process by alleviating lattice expansion. In addition, it can inhibit the generation of residual alkali on the surface of the high-nickel ternary material during storage and improve the interfacial stability between the high-nickel ternary material and the electrolyte. Conventional dry or wet-constructed cladding layers have less controllability over the thickness and conformality of the layer structure, and thus the cladding layer is usually inhomogeneous, which leads to increased ion and electron transport resistance of the electrode.
The atomic layer deposition (ALD) technique is an advanced technique for building cladding layers. This technique allows the deposition of thin films on substrates with high specific surface area, and the deposition thickness can be precisely controlled to ensure the uniformity of the deposition, even if the geometry is irregular.Yang et al. used trimethylaluminium and HF-pyridine as the precursor materials, and then used ALD to uniformly form AlF3 nanocoating layer on the surface of NCM811. The results showed that the AlF3 layer stabilised the structure of NCM811 with the lithium-nickel mixed rows.
Li et al. successfully synthesised AlF3-coated Li[Ni 0. 80Co0. 15Al 0. 05] O2 by the solution method. The original Li[Ni 0. 80Co0. 15Al 0. 05] O2 powder was first immersed in a dilute solution of Al(NO3 ) 3, and then added drop by drop into the NH4F solution to form different thicknesses of AlF3 by the precipitation reaction. Compared with the original Li[ Ni 0.8Co0.15Al 0.05 ] O2, the 0.5% AlF3 cladding samples showed higher capacity retention and multiplicity performance at different test temperatures.
1.3 Metal phosphate capping materials Metal phosphate capping materials mainly include AlPO4, MnPO4, etc. This process can inhibit the occurrence of phase transition and improve the cycling stability of ternary materials. Metal phosphates have a tendency to transform into amorphous state near the interface, and this process can inhibit the occurrence of phase transition, make the internal and interfacial structure of ternary materials more stable, and improve the cycling stability of the materials.Tang et al. successfully synthesised AlPO4-modified NCM622 by simple dry mixing and calcination, and the results of the study show that the existence forms of Al and P on the surface of NCM622 are LiAlO2 and Li 3PO4, which are composed of AlPO4 and NCM622 in the dry mixing and calcination process. The results show that the presence of Al and P on the surface of NCM622 in the form of LiAlO2 and Li 3PO4, respectively, is generated by the chemical reaction between AlPO4 and NCM622 during the calcination process, and the substitution of Al for Ni to produce the LiAlO2 and Li 3PO4 cladding layer, which stabilises the structure of NCM622 together. Although the initial discharge specific capacity at 0.1C is lower than that of the original NCM622, AlPO4 improves the cycling performance and mitigates the lattice strain at high temperatures, which improves the structural stability of the material and reduces the generation of microcracks.
Liu et al. also used AlPO4 to form a Li 3PO4-LiAlO2 capping layer on NCM811 cathode material and investigated the modification of the sample with different amounts of AlPO4 coating, and the Li 3PO4-LiAlO2 protective layer formed on the surface of NCM811 not only reduced the degradation of the layered structure near the surface to form a saline phase, but also prevented the erosion of the material by HF and H2O, which led to the structural improvement of the material. It also prevents the erosion of HF and H2O on the host material, which makes the structure of the material more stable.
Wu et al. for the first time used the non-aqueous film-forming process for the modification of AlPO4 in ternary cathode materials, which overcame the difficulty of inhomogeneity of the capping layer in the precipitation method, and the mass fraction of AlPO4 can be controlled at 0.2%, which is much lower than the content in most of the previous literature, and the ultrathin cladding layer can minimise the effect of the formation of the cladding layer on the diffusion rate of Li + , electronic conductivity and energy density, but the thinner cladding layer can also reduce the effect of the formation of the cladding layer on the diffusion rate of Li + , the electronic conductivity and energy density. The ultra-thin cladding layer can minimise the influence of the formation of the cladding layer on the Li+ diffusion rate, electronic conductivity and energy density, but the thinner cladding layer is also easier to be exhausted.
02 Electrically conductive materials
2. 1 Ionic conductive materials
The poor multiplicative performance of high nickel ternary cathode materials mainly stems from the two-dimensional diffusion pathway of Li + in the lamellar structure and the lithium-nickel mixed rows that impede Li + diffusion, which limit their application in high power density.
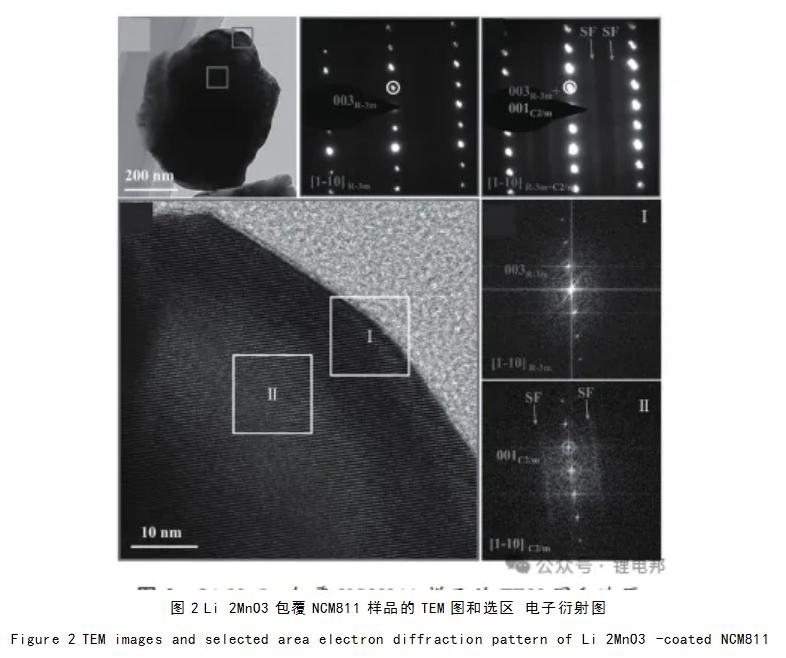
Huang et al. introduced Li 2MnO3 nanodomains into the lamellar structure of primary NCM811 particles by sol-gel method and constructed a number of domain boundaries in this integrated structure, thus forming a three-dimensional ion diffusion network.The TEM and selected electron diffraction patterns of Li 2MnO3-coated NCM811 samples are shown in Fig. 2. In this system, the reduced particle size induces a hollow structure, which increases the Li+ migration rate, and the integration of Li 2MnO3 nanodomains into the NCM811 matrix hinders the formation of lithium-nickel mixed rows. The above factors together contribute to the rapid Li+ transport, which improves the multiplicity performance of NCM811.
LiAlO2 has excellent Li + transport properties, and the LiAlO2 capping layer not only stabilises the interfacial structure between the cathode and the electrolyte, but also significantly improves the electrochemical performance as it provides a good Li + transport network for the Li + de-embedding process.Tang et al. designed an etch-induced capping layer strategy for the formation of a γ-LiAlO2 protective layer and Li + conductive cladding layer on a high-Ni NCM811 cathode. protective layer and Li+ conductive cladding layer on the high-Ni NCM811 cathode material to improve its electrochemical performance. The improved performance is mainly attributed to the diffusion of Al 3+ into the lattice interior of NCM811, which can alleviate the lithium-nickel miscibility and enhance the structural stability, while the LiAlO2 cladding layer provides a good transport network for Li + , which improves the structural stability and prevents the core material from the erosion of the electrolyte.
Li 2TiO3, with its wide operating voltage, high thermal stability and fast Li + transport kinetics, is considered as an effective cladding material for surface modification of ternary cathode.He et al. proposed a novel Li 2TiO3 nanoparticle cladding layer, which at the same time avoids the deterioration of lithium-nickel mixing and discharging process caused by Ti 4+ doping. The Li 2TiO3 nanoparticle-coated NCA8155 showed almost unchanged morphology and low surface residual bases, and thus the Li 2TiO3 capping layer significantly improved the cycling stability and multiplicity performance.
The optimum surface capping layer not only solves the instability problem by blocking the physical contact between the electrolyte and the highly active cations on the electrode surface, but also stabilises the lattice oxygen ions in the electrode and improves the Li + mobility.Wang et al. proposed a direct modulation strategy for adapting the highly active cations in the solid phase. By exploiting the stabilised oxygen vacancies and interstitials in the lithium-lanthanum-nickel-oxygen bionic conductor (layered chalcocite La4NiLiO8 ) cladding, the activity of surface lattice oxygen ions was significantly suppressed, and oxygen release from the lattice, as well as irreversible phase transitions and intergranular mechanical cracking, were inhibited. At the same time, the introduced dual ionic conductor also improves the diffusion kinetics of Li + at the particle surface and the electronic conductivity of the material body. In addition, Li et al. prepared structurally and interfacially reliable B-doped and La4NiLiO8-coated modified NCM811 cathodes for the first time using a simple one-step method.
The La4NiLiO8 coating can prevent the electrode from electrolyte corrosion and enhance the Li+ transport kinetics. In addition, B doping can effectively inhibit the harmful H2 ~ H3 phase transition and adjust the orientation of the primary particles to a radial arrangement, which prevents the generation of microcracks due to the volume change caused by the anisotropy of the crystals.
Yang et al. successfully prepared La and Al co-doped and capped modified NCM811 by a simple method, and XRD and XPS confirmed that La and Al could not only be doped into the bulk of NCM811, but also formed La2Li 0. 5Al 0. 5O4 capping layer on the surface. The improved high-voltage electrochemical performance is mainly attributed to the enhancement of the bulk structure of La and Al through co-doping, and the formation of La2Li 0. 5Al 0. 5O4 cladding layer, as a high Tc superconducting oxide, not only promotes the Li+ transport, but also protects the material from the erosion of the electrolyte. In addition, the residual lithium salt can be reduced by forming La2Li 0. 5Al 0. 5O4. The improved electrochemical performance suggests that the modification of the La2Li 0. 5Al 0. 5O4 cladding and La-Al co-doping is a competitive approach for the large-scale industrial production of NCM811 materials.
Wang et al. introduced a chalcogenide phase with a similar crystal structure to "rivet" the expansion and contraction of the layered structure, and the pinning effect significantly mitigated the deleterious structural evolution due to the volume change of the crystal structure. The lattice strain evolution per cycle is reduced by nearly 70% compared to conventional materials, which significantly enhances the integrity of the secondary particles and thus improves the reversible cycling performance of the cells. This strain suppression approach broadens the application of lattice engineering to release the strain generated by lithium embedding and paves the way for the development of high energy density cathodes with long lifetime. Wang et al. proposed a strategy to reconstruct a stable surface on nickel-rich layered oxide (NLO) cathodes using lithium- and manganese-rich layered oxide (LMR) low-strain materials. The new surface structure consists not only of a gradient structure, but also forms a defect structure rich in oxygen vacancies and cation ordering, which can simultaneously enhance the Li+ diffusion rate and stabilise the crystal structure during lithium embedding (de-embedding).These features in NLO significantly improve the electrochemical performance, especially the stability under high-voltage cycling. By introducing a spinel-like mortise and tenon structure into the layered phase of NCM811, Tan et al. were able to significantly suppress the unfavourable volume changes in the cathode material. At the same time, the mortise and tenon structure acts like a motorway for the fast Li+ transport. In addition, particles with the tongue-and-groove structure usually terminate with the most stable Cheng (003) surface. This work provides a feasible lattice work to address the stability and low first-time coulombic efficiency of NLO and helps to realise lithium-ion batteries with high energy density and long durability.
Cai et al. proposed the theory of high-pressure-induced oxygen precipitation and reported a lanthanide process to modulate the near-surface structure of the cathode material, and extended this unconventional surface modification to cobalt-depleted/cobalt-free high-energy-density layered cathodes, demonstrating that effective surface passivation inhibits surface degradation and improves electrochemical performance, and that the stability of high-pressure cycling is greatly enhanced to up to 4.8 V (vs. Li + / Li). Li). The excellent electrochemical performance stems from the reliability of the engineered surface structure and synthesis method. The engineered surface phases inhibit the oxygen precipitation reaction at high voltages. It is shown that high performance layered oxide cathode materials can be obtained by high oxygen activity passivation, selective chemical alloying and modification using strain engineering with wet chemistry.
Yang et al. prepared Li 0. 5La2Al 0. 5O4 (LLAO) in-situ cladding and Mn ion-compensated doped multilayers of LiNi 0. 82Co0. 14Al 0. 04O2. XRD refinement showed that La-Mn synergistic modification could achieve proper Li-Ni mixed rows. Calculated results and in situ XRD analyses show that the LLAO cladding is effective in suppressing mechanical cracking in the secondary particles, thanks to the suppression of internal crystal strain. Test results show that the LLAOMn-modified post-cycled cathode has a more complete morphology and less side reactions with the electrolyte. Further investigation of the cathode electrolyte interface during gas precipitation shows that NCA-LM2 releases less CO2 than NCA-P, resulting in a more stable surface.
2.2 Electrically conductive materials
Graphene has a large specific surface area, excellent electronic conductivity and mechanical properties, and its chemical properties are stable. The introduction of graphene can effectively improve the electronic conductivity and capacitance of the surface of electrode materials.
Luo et al. prepared a NCA8155/graphene composite material (GNCA8155) with three-dimensional nanostructure by template self-assembly method. Firstly, the prepared graphene was dissolved in anhydrous ethanol, and a homogeneous graphene dispersion was formed under high-power ultrasonic stirring, then NCA8155 powder was added to the above solution, and the mixture was self-assembled by slight ultrasonic stirring, and then dialysed and dried to obtain G-NCA8155. The three-dimensional network of graphene increases the specific surface area of the material, and the synergistic effect can increase the electronic conductivity and stabilise the crystal structure, thus significantly improving the multiplicity performance and capacitance. The three-dimensional graphene network increases the specific surface area of the material, while the synergistic effect improves the electronic conductivity and stabilises the crystal structure, thus significantly improving the multiplicity performance and cycling stability.
Tian et al. prepared a three-dimensional porous graphene aerogel-encapsulated NCM622 nanoparticles ( NCM@ GA) with a unique structure by co-precipitation and hydrothermal reaction. Due to its high electrical conductivity and a large number of interwoven open pore structures, the self-assembled graphene aerogel network can greatly accelerate the electron and ion transport rates and enhance the electrochemical reaction kinetics. In addition, the well-dispersed NCM622 nanoparticles can provide a larger electrode-electrolyte interface and facilitate the rapid Li+ transport. Therefore, the synergistic effect of the three-dimensional conductive structure and well-dispersed nanoparticles can effectively enhance the electrochemical performance of NCM@ GA materials.
Liu et al. used sucrose and glucose as carbon materials to construct a carbon nanoclayer on the surface of NCA8155. The results showed that the cladding layer constructed with sucrose as the carbon source has better electrochemical performance. The reason is that the carbon layer formed by glucose is denser, while the one formed by sucrose is looser with larger-sized pores. Such carbon cladding layer has a larger specific surface area, which is favourable for the infiltration of electrolyte, and also facilitates the de-embedding and migration of Li + and slows down the erosion of the electrolyte on the host material.
Cao et al. prepared a bifunctional conductive polymer combining the excellent electronic conductivity of polyaniline (PANI) and the high ionic conductivity of poly(ethylene glycol) (PEG) for surface modification of NCM811 to obtain high-performance NCM@PANI-PEG composites. The highly elastic and flexible PANI-PEG polymer plays a crucial role in mitigating the volume shrinkage and swelling of NCM811 during cycling. Dissolution of the transition metal is caused by HF corrosion from electrolyte decomposition, which adversely affects the electrochemical performance. The dissolution of Ni, Co and Mn transition metals in the surface-modified electrodes was lower than in the unmodified electrodes for the same storage time. The PANI-PEG protective layer encapsulated onto the surface of the NCM811 particles provided a physical barrier to prevent the cathode material from being attacked by HF, which inhibited the dissolution of the transition metals and thus improved the cycling stability of the host material at elevated temperatures.
Perylene-3,4,9,10-tetracarboxylic dianhydride ( PTCDA) is an N-type organic semiconductor material with high chemical stability.The high electron affinity ( ~3. 6 eV) of PTCDA leads to the easier stabilisation of the additional negative charge, providing a theoretical basis for the adhesion to graphene. Structurally, the PTCDA molecule is a two-dimensional conjugated π-electron system, and the benzene ring it carries is consistent with the hexagonal structure of graphene.Ning et al. prepared homogeneous rGO-coated NCM811 (PG-NCM) materials by simple physical mixing in the presence of PTCDA. The synergistic effect of rGO nanosheets and PTCDA provides better electronic conductivity and more stable electrode-electrolyte interface when using PG-NCM as cathode material. In particular, P1G1-NCM (containing only 1.0% by mass additive) showed the best performance among all the samples, achieving a specific capacity of 194.1 mAh-g-1 at 1C, a capacity retention of 92.8% after 100 cycles, and an improved performance at higher multiplicities (122.1 mAh-g-1 at 10C).
03 Cladding Materials
3.1 Electronic conductive materials and metal oxide composite cladding
A composite cladding of an electrically conductive material and a metal oxide can simultaneously improve the electrical conductivity and structural stability of the cathode material. In this approach, one component improves the cycling performance by protecting the surface from unwanted side reactions, while the other enhances the electronic conductivity and increases the specific capacity of discharge.Y2O3 is used as the cladding agent, and graphene is used as the conductive additive.Y2O3 belongs to the rare-earth element oxides, which are highly thermally stable.Loghavi et al. modified the NCA8 with Y2O3 by a wet chemical calcination method. Loghavi et al. modified NCA811 cathode material with Y2O3 by wet chemical calcination and mechanically mixed the prepared material with graphene. The electrochemical performance tests showed that the NCA811, Y2O3 / NCA811 and graphene / Y2O3 / NCA811 materials provided discharge specific capacities of 109, 136 and 164 mAh-g-1 at 2C multiplicity, respectively. The graphene/Y2O3/NCA811 material still has a specific capacity of 180 mAh-g-1 after 100 cycles (0.5C), while the original NCA811 only provides 87 mAh-g-1 .
3. 2 Composite cladding of ionic and electronic conductive materials
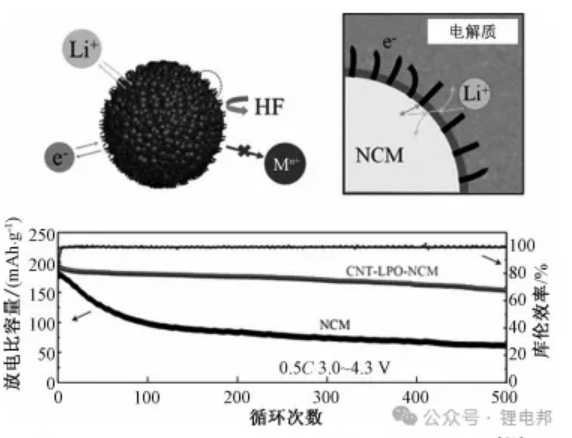
Constructing a bifunctional cladding layer with high ionic and electronic conductivity on the surface of the material improves the stability of the battery during the cycling process and the ion storage process. The body material, ionic cladding material, electronic cladding material and electrolyte together form a four-phase cathode-electrolyte interface, which plays a key role in the substantial increase in capacity retention.Yang et al. constructed a multifunctional cladding layer with high ionic and electronic conductivity on the surface of NCM811 to improve the stability of the battery during cycling. Phosphoric acid reacts with the residual lithium salts on the original NCM811 to form a Li 3PO4 cladding layer with carbon nanotube penetration, which has high ionic and electronic conductivity.The NCM811, Li 3PO4, CNT, and the electrolyte form a four-phase cathode-electrolyte interface, which is crucial for the improvement of capacity retention rate.The capacity retention rate increases from 50.3% to 50.3% after 500 cycles at 0.5C multiplicity. After 500 cycles at 0.5C multiplier, the capacity retention rate increased from 50.3% to 84.8%. The improved NCM811 still exhibits excellent electrochemical performance at a high cutoff voltage of 4.5 V, a high temperature of 55 °C, and a high multiplication rate of 10 C. In addition, the NCM811 also exhibits excellent electrochemical performance in high humidity air. In addition, after 2 weeks of exposure to high humidity air, it can provide a discharge specific capacitance of 154.2 mAh-g-1 after 500 cycles.The mechanical schematic and cyclic performance of the CNT-LPO-NCM is shown in Fig. 3.
In order to improve the ionic and electronic conductivity of cathode materials for lithium-ion batteries, Na et al. proposed a surface modification of Li 1. 03 (Ni 0. 88Co0. 08Mn0. 04) O2 cathode materials with high ionic conductor Li 1. 3Al 0. 3Ti 1. 7 [ PO4 ] 3 (LATP) and high electronic conductor multi-walled carbon nanotubes, and LATP powders were prepared by a modified Pechini method. LATP powders were prepared using the modified Pechini method. The high ionic and electronic conductive network on the surface of the surface-modified high-Ni NCM electrodes enables fast Li+ and electron transport, which significantly enhances the electrochemical performance during charge/discharge cycling.
3.3 Ionically Conductive Materials and Metal Oxide Composite Coatings
Metal oxides can protect the material from electrolyte erosion, and the metal oxide cladding can improve the stability of the material interface structure and enhance the cycling performance of the battery. The ionic conductive material cladding layer can enhance the Li+ transport ability and improve the multiplication performance of the battery. The composite coating layer of ionic conductive materials and metal oxides can simultaneously improve the capacity retention and multiplication performance of the battery.
Maiti et al. employed a simple and effective ALD capping strategy by surface capping the NCM424 powder material with Al 2O3, Li 5AlO4, and Na5AlO4, which enhanced its redox activity and inhibited the irreversible oxygen release from the lattice. After more than 400 cycles at 1C multiplicity, the specific capacity of the uncapped NCM424 material was only 63 mAh-g-1, whereas the NCM424 material with the composite cladding layer showed approximately twice the specific capacity. Analyses of XPS spectra and voltage distributions revealed that the surface manganese of the modified NCM424 material was partially reduced from the tetravalent state to a lower valence state. According to the findings, the reduction of surface manganese in the presence of ALD cladding may occur due to the contact of trimethylaluminium volatiles with them through their decomposition reaction on the surface of the cathode material. The key finding of this work is that the charge capacity delivered by cationic oxidation ( Ni 2+ / Ni 4+ ,Co 3+ / Co 4+ ) and confirmed by anionic oxidation is slightly lower than that of the uncoated material compared to all the capped cathode materials. This finding may be related to the modified electrode-electrolyte interface formed on the surface capping layer of NCM424 particles.
04
High-nickel ternary cathode materials have attracted much attention from researchers because of their high energy density. However, these materials have a series of problems, such as sensitivity to air, reaction with electrolyte, cation mixing and exclusion, lattice oxygen precipitation, transition metal ion migration, and microcrack formation, which limit the application and development of high-nickel ternary cathode materials. Coating is an important method for surface modification of high nickel ternary cathode materials. Commonly used cladding materials include electrochemical inert materials, ionic conductive materials, and electronic conductive materials. On the basis of this development to the composite coating, common composite coating has electronic conductive material and metal oxide composite coating, ionic conductive and electronic conductive material composite coating, ionic conductive material and metal oxide composite coating. Coating materials can not only protect the surface of the material and improve the structural stability of the material, but also improve the transport capacity of ions or electrons and improve the electrochemical properties of the material. However, the current cladding materials still have great problems, for example, with the embedding and detachment of Li + during the battery operation, the inconsistency of expansion and contraction between the cladding layer and the body material will bring about a large degree of mismatch between the materials, which will cause the cladding material to fall off. Therefore, the interfacial stability between the cladding layer and the host material needs to be further addressed in the future.
Note: This article comes from Lithium BONUS. Non-Yongbang views, disclaimer this website number based on the purpose of sharing reproduced, reproduced the copyright of the article belongs to the original author or the original public number, if there is any infringement involved please inform us in a timely manner, we will be verified and deleted. The purpose of publishing this article is to convey more industry information, for reference only, does not mean that Yongbang is responsible for its views and content. This article does not constitute any investment advice, invest accordingly at your own risk.
Previous Page
Latest News









